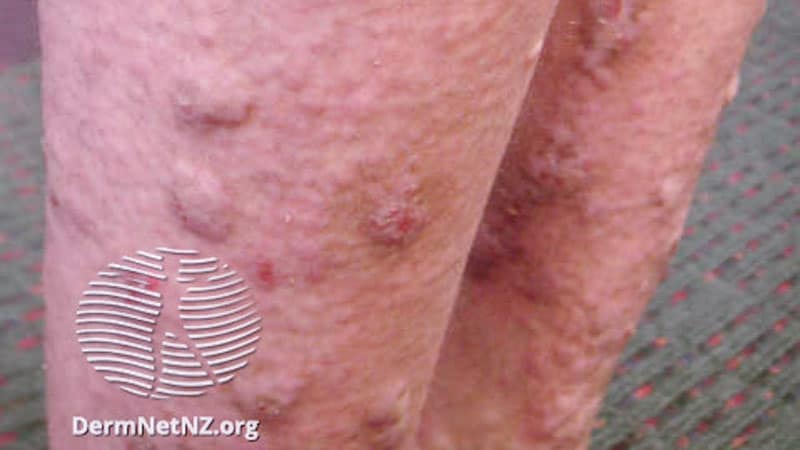
BOSTON ― Dupilumab, a human monoclonal IgG4 antibody, was an effective treatment for prurigo nodularis (PN), improving itching and skin lesions after 12 and 24 weeks of treatment, in a phase 3 trial presented at the American Academy of Dermatology (AAD) 2022 Annual Meeting.
There are currently no US Food and Drug Administration–approved systemic therapies for PN. Although several treatments for the disease are used off label for the condition, such as ultraviolet light therapy and immunosuppressive agents, moderate to severe PN is usually difficult to control, noted Gil Yosipovitch, MD, director of the Miami Itch Center at the University of Miami Miller School of Medicine in Florida. He led the research and presented the findings at the conference.
“Many dermatologists feel very uncomfortable dealing with these patients because they suffer from chronicity, they are miserable, and previously, the drugs didn’t work well,” Yosipovitch told Medscape Medical News. The results from this trial “are very promising,” he said. “It opens a new field of treatment for itchy conditions.”
The trial, named LIBERTY-PN PRIME2, enrolled patients aged 18 to 80 who had been living with PN for at least 3 months. Patients had at least 20 lesions at baseline as well as severe itch, defined as a score of 7 or greater on the Worst Itch Numerical Rating Scale (WI-NRS). The scale ranges from 0 (no itch) to 10 (worst itch imaginable). Participants also had a history of treatment failure with medium to super-potent topical corticosteroids (TCSs), or treatment with TCSs was not medically advisable for them.
The randomized, double-blinded study enrolled 160 adults with PN. Of those, 78 were assigned to the treatment arm and received a 600-mg loading dose of dupilumab, administered subcutaneously, followed by 300-mg doses every 2 weeks for 24 weeks; 82 patients were allocated to receive placebo.
During the study, 25 patients in the placebo arm discontinued treatment. In the treatment arm, one patient was not treated and two discontinued treatment because of lack of efficacy.
The primary endpoint of the study was a reduction of at least 4 points on the WI-NRS at 12 weeks. Secondary endpoints included at least a 4-point WI-NRS reduction at 24 weeks and clear to nearly clear skin, defined as having a score of 0 or 1 on the Investigator’s Global Assessment PN-Stage (IGN PN-S). The scale ranges from 0 (clear) to 4 (severe).
At 12 weeks, 37.2% of patients given dupilumab reported a reduction of at least 4 points in WI-NRS, compared to 22.0% of patients given placebo (P = .0216). By 24 weeks, 57.7% of adults who received dupilumab achieved a ≥4-point reduction in WI-NRS, compared to 19.5% of those who received placebo (P < .0001). Additionally, 44.9% of participants in the treatment arm achieved a score of 0 or 1 on the IGA PN-S, compared to 15.9% of those in the placebo arm (P < .0001).
Forty-four participants who received dupilumab (57.1%) and 42 participants who received placebo (51.2%) reported at least one treatment-emergent adverse event (TEAE) during the study, though none of these events were serious. The most common TEAE in the study was headache, occurring in five patients taking placebo and four patients receiving dupilumab. In the dupilumab group, there were five cases of herpes virus infection, four non-herpes skin infections, and three cases of conjunctivitis. In the placebo group, seven non-herpes skin infections were reported.
Sanofi and Regeneron, who jointly developed dupilumab, plan to file for regulatory approval for dupilumab for PN “around the world” in the first half of this year, according to a press release.
“It’s great news and a step in the right direction,” Sarina Elmariah, MD, PhD, a dermatologist at Massachusetts General Hospital and instructor of dermatology at Harvard Medical School in Boston, told Medscape Medical News. She was not involved with the research.
“We’re finally starting to shed light on this condition and its pathogenesis,” she said. She noted that other potential therapeutics for PN are also in development. “It’s reflective of the fact that we are making strides in this area.”
American Academy of Dermatology (AAD) 2022 Annual Meeting.
Sanofi and Regeneron Pharmaceuticals, Inc, sponsored the LIBERTY-PN PRIME2 trial.
Yosipovitch has reported financial relationships with Bellus Health, Eli Lilly, Galderma, GSK, Kiniksa Pharmaceuticals, LEO Pharma, Novartis, Pfizer, Regeneron, Sanofi, and Trevi Therapeutics. Elmariah is on the advisory boards of Sanofi, Galderma, and Trevi Therapeutics.
For more news, follow Medscape on Facebook, Twitter, Instagram, and YouTube.