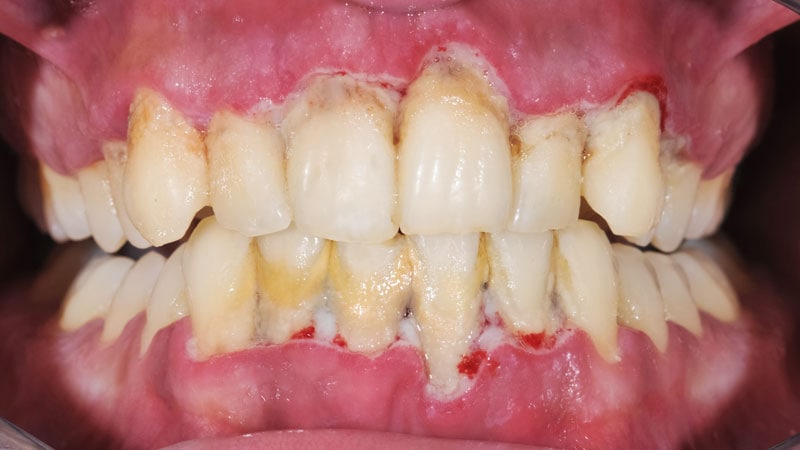
An oral, experimental medication that targets the bacteria that causes gum disease may offer a “new treatment paradigm” for mild to moderate Alzheimer’s disease (AD), new research suggests.

Dr Michael Detke
Results from the phase 2/3 GAIN trial of atuzaginstat (Cortexyme Inc), which targets the gum bacteria Porphyromonas gingivalis (Pg), suggest the pathogen is a “potential driver of AD.”
“We showed the efficacy and safety of our drug in patients with mild to moderate AD who have Pg infection; and we think we found an optimal dose. This represents a whole new treatment paradigm for AD,” study investigator Michael J. Detke, MD, PhD, chief medical officer at Cortexyme, told Medscape Medical News.
The findings were presented at the 14th Clinical Trials on Alzheimer’s Disease (CTAD) Conference.
Low-Grade Chronic Infection
Pg is associated with gum disease, including gingivitis and periodontal disease. Detke added that periodontal disease affects about 65 million Americans.
Previous research has linked periodontal disease with an increased risk for AD. One study cited by Detke showed individuals with a severe form of the gum disease declined by six points on the AD Assessment Scale-Cognitive subscale (ADAS-Cog) in 6 months vs only one point among those with mild or no periodontal disease.
Evidence linking inflammation with AD is “consistent with the idea there’s…a low-grade chronic infection that’s causing this inflammatory response,” Detke said.
He noted that 22 of the last 25 genetic risk factors identified for AD relate to immune system function.
Pg is different from other bacteria in that it gets inside cells and relies on proteins as an energy source instead of sugars or carbohydrates. The bacteria release proteases called gingipains that “chop up” proteins into fragments that provide the energy, said Detke.
Atuzaginstat is designed to stop damage caused by gingipain protease by cutting off the bacteria’s food supply. Because Pg is inside cells and “can go dormant or develop resistance,” it is extremely difficult to completely wipe it out with typical antibiotics, Detke noted.
Slowed Cognitive Decline
The GAIN trial included 643 generally healthy patients aged 55 to 80 years (mean age, 69 years) with mild to moderate AD, as measured by a score of 12 to 24 on the mini-mental state exam. About 65% were ApoE4 carriers.
All participants were randomly assigned to receive placebo or a low dose (40 mg twice a day) or high dose (80 mg twice a day) of atuzaginstat for 48 weeks.
In addition to standard cognitive outcomes, researchers assessed biomarkers of Pg in saliva, blood, and cerebrospinal fluid (CSF).
The two co-primary endpoints were scores on the ADAS-Cog and AD Cooperative Study–Activities of Daily Living (ADCS-ADL). Results showed that neither of these outcomes were significant in the overall intent-to-treat cohort.
However, when investigators examined only participants with worse Pg infection, there was a correlation between lowering Pg levels and better clinical outcome.
“In people with high levels of Pg in saliva, in serum, and CSF — in all of these — you saw significant slowing of decline on ADAS-Cog — by anywhere from 26% to 57%,” Detke said.
Another statistical analysis showed slowing of cognitive decline by 40% to 56%.
“From the AD literature, 20% or 30% is good, so seeing 40% or 50% is really more than anything anyone has seen, especially in this difficult mild-to-moderate population,” he added.
A Causal Pathogen?
There were also correlations between Pg levels in saliva at 24 weeks and clinical outcomes at both 24 weeks and 48 weeks. “If Pg is causal, then change at 24 weeks might predict clinical impact at both timepoints and that’s what we saw,” with results being “pretty highly significant,” said Detke.
“There are almost no other AD studies that have shown a correlation between a biomarker and clinical outcome,” he noted.
Looking at bilateral hippocampal volume, the investigators also found the drug slowed atrophy — by 22% in the lower dose group and by 11% in the higher dose group. Efficacy was comparable for the 40 mg and 80 mg doses.
There was little difference between men and women, APOE carriers and noncarriers, or between patients with mild or moderate disease. “It looks like it’s working across everyone who’s got a high level of infection,” said Detke.
The most common side effects were gastrointestinal (GI), including nausea and diarrhea. This makes sense as Pg is found in the GI tract and likely affects the microbiome to some degree, Detke noted.
Overall, the rates of serious adverse events (SAEs) were too few to draw conclusions, he said. However, SAEs were reported in 19 members in the placebo group, 20 in the low-dose group, and 25 in the high-dose group.
There were two cases of elevated bilirubin, both of which were in the high-dose group. There were also five deaths in the high-dose group, but they were not deemed to be related to the drug.
There were no concerns related to amyloid-related imaging abnormalities (ARIAs), “which is a big advantage over amyloid drugs,” said Detke.
The drug does not clear the bacteria entirely, he noted. Given to animals infected with Pg, it “knocks down levels of the bacteria by 80% to 90%, but doesn’t eradicate it,” he added. “So this is probably a rest-of-life chronic treatment” akin to a protease inhibitor used to treat HIV, Detke said.
More Research Needed
Commenting on the research for Medscape Medical News, Rebecca Edelmayer, PhD, senior director of scientific engagement at the Alzheimer’s Association, said it is “encouraging” to see approaches that look at the disease from new angles.
“We learn something new from every trial,” said Edelmayer.
“Research into this particular approach should continue and we look forward to seeing how this drug performs in future clinical trials,” she added.
The study was funded by Cortexyme Inc. Detke is a full-time employee of Cortexyme and holds equity in the form of stock options. Edelmayer has disclosed no relevant financial relationships.
14th Clinical Trials on Alzheimer’s Disease (CTAD) Conference: Late-breaking Readout Roundtable #6. Presented November 11, 2021.
For more Medscape Neurology news, join us on Facebook and Twitter.