Overview
Local anesthetics provide a reversible regional loss of sensation. Local anesthetics reduce pain, thereby facilitating surgical procedures. Delivery techniques broaden the clinical applicability of local anesthetics. These techniques include topical anesthesia, infiltrative anesthesia, ring blocks, and peripheral nerve blocks (see the Technique section below for links to detailed, illustrated articles demonstrating these techniques).
Local anesthetics are safer than general or systemic anesthetics; therefore, they are used whenever possible. In addition, they are relatively easy to administer and readily available. Local anesthetics have been undergoing development for centuries, and, as this article illustrates, research continues to provide surgeons with pharmacologic variety and to provide patients with anesthetic agents that have superior safety and efficacy profiles.
Background
Although the medical world cannot cure every disease, the control of pain to ensure patient comfort should be a goal. In 1860, cocaine, the oldest anesthetic, was extracted from the leaves of the Erythroxylon coca bush. In 1884, Sigmund Freud and Karl Koller were the first to use it as an anesthetic agent during ophthalmologic procedures.
Procaine, a synthetic alternative to cocaine, was not developed until 1904. Procaine is an ester of para-aminobenzoic acid (PABA). As procaine is metabolized, PABA, a known allergen, is released as a metabolic product. The potential for severe allergic reactions limits the use of procaine and other ester-type anesthetic agents. Tetracaine, another ester-type anesthetic, was introduced in 1930. Tetracaine is more potent than procaine, and it causes similar allergic reactions.
In 1943, an alternative class of anesthetics was discovered when Lofgren developed lidocaine. This agent is an amide derivative of diethylaminoacetic acid, not PABA; therefore, it has the benefit of a low allergic potential. Since then, multiple amide-type anesthetics have been introduced into clinical use. Slight chemical alterations to the compounds have imparted beneficial characteristics, including increased duration and potency, to each. These compounds offer the surgeon more choices, and anesthetics can be appropriately matched to different procedures.
Pathophysiology
Reviewing the physiology of nerve conduction is important before any discussion of local anesthetics. Nerves transmit sensation as a result of the propagation of electrical impulses; this propagation is accomplished by alternating the ion gradient across the nerve cell wall, or axolemma.
In the normal resting state, the nerve has a negative membrane potential of -70 mV. This resting potential is determined by the concentration gradients of 2 major ions, Na+ and K+, and the relative membrane permeability to these ions (also known as leak currents). The concentration gradients are maintained by the sodium/potassium ATP pump (in an energy-dependent process) that transports sodium ions out of the cell and potassium ions into the cell. This active transport creates a concentration gradient that favors the extracellular diffusion of potassium ions. In addition, because the nerve membrane is permeable to potassium ions and impermeable to sodium ions, 95% of the ionic leak in excitable cells is caused by K+ ions in the form of an outward flux, accounting for the negative resting potential. The recently identified 2-pore domain potassium (K2P) channels are believed to be responsible for leak K+ currents.
When a nerve is stimulated, depolarization of the nerve occurs, and impulse propagation progresses. Initially, sodium ions gradually enter the cell through the nerve cell membrane. The entry of sodium ions causes the transmembrane electric potential to increase from the resting potential. Once the potential reaches a threshold level of approximately -55 mV, a rapid influx of sodium ions ensues. Sodium channels in the membrane become activated, and sodium ion permeability increases; the nerve membrane is depolarized to a level of +35 mV or more.
Once membrane depolarization is complete, the membrane becomes impermeable to sodium ions again, and the conductance of potassium ions into the cell increases. The process restores the excess of intracellular potassium and extracellular sodium and reinstates the negative resting membrane potential. Alterations in the nerve cell membrane potential are termed the action potential. Leak currents are present through all the phases of the action potential, including setting of the resting membrane potential and repolarization.
Mechanism of action
Local anesthetics inhibit depolarization of the nerve membrane by interfering with both Na+ and K+ currents. The action potential is not propagated because the threshold level is never attained.
Although the exact mechanism by which local anesthetics retard the influx of sodium ions into the cell is unknown, 2 theories have been proposed. The membrane expansion theory postulates that the local anesthetic is absorbed into the cell membrane, expanding the membrane and leading to narrowing of the sodium channels. This hypothesis has largely given way to the specific receptor theory. This theory proposes that the local anesthetic diffuses across the cell membrane and binds to a specific receptor at the opening of the voltage-gated sodium channel. The local anesthetic affinity to the voltage-gated Na+ channel increases markedly with the excitation rate of the neuron. This binding leads to alterations in the structure or function of the channel and inhibits sodium ion movement. Blockade of leak K+ currents by local anesthetics is now also believed to contribute to conduction block by reducing the ability of the channels to set the membrane potential.
On the basis of their diameter, nerve fibers are categorized into 3 types. Type A fibers are the largest and are responsible for conducting pressure and motor sensations. Type B fibers are myelinated and moderate in size. Type C fibers, which transmit pain and temperature sensations, are small and unmyelinated. As a result, anesthetics block type C fibers more easily than they do type A fibers. Therefore, patients who have blocked pain sensation still feel pressure and have mobility because of the unblocked type A fibers.
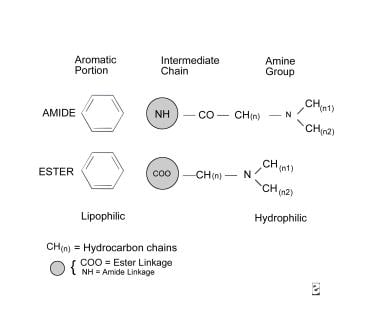
All local anesthetics have a similar chemical structure, which consists of 3 components: an aromatic portion, an intermediate chain, and an amine group (see molecular diagram below). The aromatic portion, usually composed of a benzene ring, is lipophilic, whereas the amine portion of the anesthetic is responsible for its hydrophilic properties. The degree of lipid solubility of each anesthetic is an important property because its lipid solubility enables its diffusion through the highly lipophilic nerve membrane. The extent of an anesthetic’s lipophilicity is directly related to its potency.
Molecular diagram.
Local anesthetics are weak bases that require the addition of hydrochloride salt to be water soluble and therefore injectable. Salt equilibrates between an ionized form and a nonionized form in aqueous solution. Equilibration is crucial because, although the ionized form is injectable, the nonionized base has the lipophilic properties responsible for its diffusion into the nerve cell membrane. The duration of action of an anesthetic or the period during which it remains effective is determined by its protein-binding activity, because the anesthetic receptors along the nerve cell membrane are proteins.
The intermediate chain, which connects the aromatic and amine portions, is composed of either an ester or an amide linkage (see molecular diagram above). This intermediate chain can be used in classifying local anesthetics.