Overview
Botulinum neurotoxin is the most poisonous substance known. If inhaled, 1 ug would kill a person.
It exerts its effect by paralyzing striated muscles or the autonomic-innervated muscles. The muscle paralyzing feature of botulinum toxin, when used beneficially, has proven to be useful in more than 50 pathological conditions, including cosmetic applications.
Today, botulinum neurotoxin injection is the most commonly performed cosmetic procedure in the world. See the images below.
Before BOTOX® treatment. Image courtesy of Allergan, Inc.
After BOTOX® treatment. Image courtesy of Allergan, Inc.
Botulinum toxin is a polypeptide produced by the gram-positive anaerobic bacterium Clostridium botulinum.
Eight serologically distinct botulinum neurotoxins exist, designated as A, B, C1, C2, D, E, F, and G. Seven are associated with paralysis. Types A, B, E and, rarely, F and G are associated with human botulism.
Botulism is a bilaterally symmetric descending neuroparalytic illness caused by botulinum neurotoxin. The German physician and poet Justinus Kerner published the first full description of clinical symptoms of food-borne botulism from 1817-1822.
His observations followed an increase in food poisoning in Stuttgart from 1795-1813 caused by general economic hardship related to the Napoleonic wars and a decline in hygienic measures of food production and handling. The illness became known as “sausage poisoning” because it was observed to follow ingestion of spoiled sausage. The word botulism comes from the Latin botulus, meaning sausage.
Kerner deduced that the toxin acts by interrupting signal transmission within the peripheral and sympathetic nervous system, leaving sensory transmission intact. He also hypothesized possible therapeutic uses of the sausage toxin. In 1895, the microbiologist Emile-Pierre van Ermengen discovered the association with an anaerobic bacterium during an outbreak of botulism following a funeral ceremony in the Belgian village of Ellezelles.
When foods tainted with neurotoxin are ingested, the neurotoxin is absorbed and spread hematogenously to peripheral cholinergic nerve terminals, where it blocks the release of acetylcholine. The neurotoxin is heat labile and denatured by cooking. Sporadic outbreaks of botulism in the United States occur after ingestion of home-canned foods, meat products, and preserved fish. The incubation period following ingestion is 18-36 hours.
In contrast, infant botulism is caused by colonization of the gut by C botulinum, and subsequent production and absorption leads to absorption of the toxin. Honey consumption has been implicated in infant botulism, and microbiologic surveys have identified clostridial spores (mostly type B) in up to 25% of honey products.
Wound botulism may occur if the organism infects a wound and produces the toxin. The clinical syndrome of botulism is one of progressive muscle weakness, often beginning in the extraocular or pharyngeal muscles and becoming generalized. GI tract complaints may be prominent. Dilated unreactive pupils are common, and mucous membranes are often dry and erythematous. No sensory signs are associated, and alertness is maintained as long as respiration is adequate.
In 1946, Schantz helped isolate botulinum toxin type A in crystalline form. In the early 1970s, Scott experimented with botulinum toxin type A in monkeys for the treatment of strabismus. In 1977-1978, he performed trials in patients with strabismus. In the mid-1980s, he treated an individual with botulinum toxin for cosmetic reasons. Carruthers, Carruthers, Brin, and the Columbia University group noticed cosmetic improvement following botulinum toxin injection for facial dystonias and began pursuing this line of investigation in the late 1980s and early 1990s.
Botulinum toxins currently are used to treat various disorders, including strabismus, hemifacial spasms, focal dystonias (eg, blepharospasm, torticollis, spasmodic dysphonia, limb dystonia, writer’s cramp), spasticity, tremor, tics, synkinesis, hyperhidrosis, achalasia, and sphincter dysfunction. They are being evaluated to treat headaches and pain syndromes.
Botulinum toxins approved by the US Food and Drug Administration (FDA) for aesthetic surgery include the following:
OnabotulinumtoxinA (BOTOX® Cosmetic) – Moderate to severe glabellar lines, moderate to severe lateral canthal lines (ie, crow’s feet), moderate to severe forehead lines manifested through frontalis muscle activity
AbobotulinumtoxinA (Dysport®) – Moderate to severe glabellar lines
IncobotulinumtoxinA (Xeomin®) – Moderate to severe glabellar lines
PrabotulinumtoxinA (Jeuveau®) – Moderate to severe glabellar lines
Mechanism of action
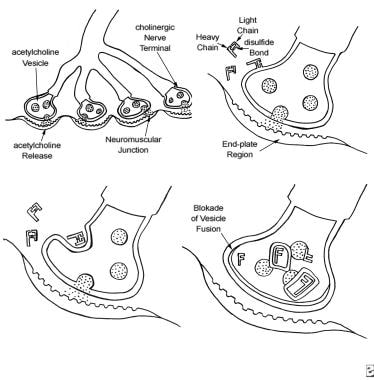
Botulinum toxins block acetylcholine release, causing a chemical denervation. Neurotransmission at the neuromuscular junction involves the release of acetylcholine from the presynaptic nerve terminal. Acetylcholine release requires docking and binding of the neurotransmitter vesicles to the presynaptic membrane.
Several different proteins mediate this process. N -ethylmaleimide-sensitive fusion protein (NSF) is a cytoplasmic protein that is part of the fusion complex. Soluble N -ethylmaleimide-sensitive fusion–attachment proteins (SNAPs) are found in the cytoplasm and serve as attachment and stabilizing proteins for the NSF complex. SNAP receptors (SNAREs) are found on the vesicle and plasma membranes. SNAREs include vesicle-associated membrane protein (VAMP/synaptobrevin) and the plasma proteins SNAP-25 and syntaxin.
Botulinum toxin is a zinc-dependent endopeptidase made up of a light (50 kilodaltons [kDa]) and a heavy (100 kDa) chain linked by disulfide bonds.
See the image below.
The 4-step process by which botulinum toxin reduces neuromuscular activity: (a) Normally functioning neuromuscular junction; (b) Binding step: The binding of botulinum dichain as the 100-kDa heavy chain binds to the cholinergic site on the cell membrane of the presynaptic cholinergic motor nerve terminal at a neuromuscular junction; (c) Internalization: The invagination of the cell membrane around the toxin molecule produces small endocytic vesicles within the cytoplasm of a motor nerve terminal; (d) Translocation step: Penetration and translocation of the neurotoxin 30-kDa light chain domain across the endosomal membrane of the endocytic vesicle into the cytosol of the motor nerve terminal; Blocking step: The neurotoxin 50-kDa light chain domain impedes the fusion of the acetylcholine vesicles on the inner side of the nerve terminal plasma membrane and the exocytosis of acetylcholine and its release into the synaptic cleft, preventing muscle contraction (Bendetto AV, 1999).
The mechanism of action includes the following 4 key steps:
The first step is binding of the toxin to specific receptors on the surface of the presynaptic cell surface, mediated by the C-terminal half of the heavy chain. This step occurs over approximately 30 minutes.
The second step is internalization, an energy-dependent receptor-mediated endocytic process. In this step, the plasma membrane of the nerve cell invaginates around the toxin-receptor complex, forming a toxin-containing vesicle inside the nerve terminal.
The third step is translocation. After internalization, the disulfide bond is cleaved, and the 50-kDa light chain of the toxin molecule is released across the endosomal membrane of the endocytic vesicle into the cytoplasm of the nerve terminal.
The final step is blocking. The 50-kDa light chain of serotypes A and E inhibit acetylcholine release by cleaving a cytoplasmic protein (SNAP-25) required for the docking of acetylcholine vesicles on the inner side of the nerve terminal plasma membrane. Botulinum toxin type D is specific for VAMP/synaptobrevin. Botulinum toxin types B and F also affect the VAMP/synaptobrevin protein. These actions impede the release of acetylcholine into the synaptic cleft.
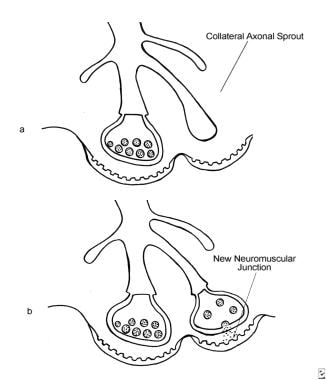
The clinical effect of botulinum toxin injections lasts 2-6 months and then resolves. Once chemical denervation begins, axon terminals form new unmyelinated sprouts, and the motor endplate regions expand. See the image below.
The development of extrajunctional acetylcholine receptors and expansion of the motor endplate occur after an injection of BOTOX®. (a) An axon terminal proliferating external collateral sprouts. (b) A single nerve sprout reestablishing a new neuromuscular junction results in the return of muscle activity (Bendetto AV, 1999).
After several months, the inactivated terminals slowly recover function, and the new sprouts and end plates regress. Recovery of inactivated terminals appears to be the basis of the loss of clinical effect several months after injection.